
|
Fe8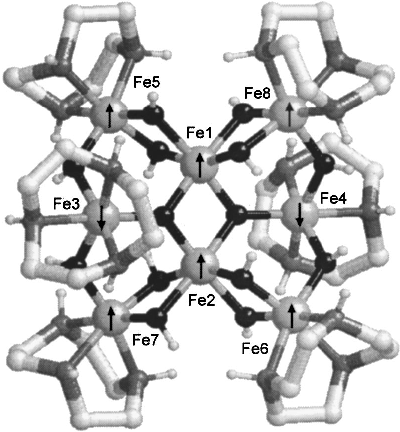 [(tacn)6Fe8O2(OH)12]8+[Br8.9H2O]8- where tacn (pronounce tack-en) stands for 1,4,7-Triazacyclononane and is the distorted ring with three N H units (small, gray - tiny, white). Carbon atoms are (small, white) and come in twos, sharing a double bond. Fe is (large, gray) and O is (small, black). All Fe are Fe3+, high spin state, {$S=\frac 5 2$}. The total effective spin, in the depicted ground state configuration is therefore {$S_{tot}=(6-2)\frac 5 2 = 10$} For µSR purposes it does have three unsaturated bonds per tacn, i.e. eighteen in all. Certain to produce radicals: by eye I would count the following isomers:
If the symmetry is lower than I assumed there are certainly two mirror planes (horizontal and vertical, so that at most there are six isomes with multiplicity: 4 4 4 2 2 2 |